Toxicology and Safety Pharmacology
Lower Risk Development Stems From Ncardia
When you need to increase confidence in your preclinical toxicology and safety pharmacology studies, partner with Ncardia, the proven iPSC experts.
Cardiotoxicity Testing
Cardiotoxicity is a common cause of late-stage failures or postmarket withdrawals, making iPSC-derived cardiovascular cells essential tools for predicting and assessing cardiac risk before clinical trials.
Cardiac Toxicology and Safety Pharmacology Assays
We offer a range of cardiac toxicology and safety assays — including custom assays — using iPSC-derived cardiomyocytes, enabling comprehensive evaluation of drug-induced heart effects in support of regulatory compliance and safer drug development:
- Assays can be multiplexed for functional and structural cardiotoxicity assessments
- Noninvasive assays allow both acute and chronic studies
| Proliferation, adhesion & cell loss | IncuCyte, ImageXpress Confocal, xCELLigence RTCA Cardio, CardioExcyte 96, Cytation |
|---|---|
| Gene expression | BioRad Opus 384 qPCR, RNAseq, whole genome sequencing |
| Protein expression | Western Blot, Spark Multimode Microplate Reader, NovoCyte Flow Cytometer, ImageXpress Confocal, MSD MESO Sector 600MM, Cytation |
| E-phys (MEA) | Maestro MEA, CardioExcyte 96, LUMOS |
| Ca2+ transients | FDSS µCell |
| Contractility | xCELLigence RTCA Cardio, CardioExcyte 96 |
| Metabolism assays | Spark Multimode Microplate Reader (w/ Agilent MitoXpress), ImageXpress Confocal, Cytation (w/ mitochondrial dyes) |
Neurotoxicity Assessments
Neural tissues are complex, sensitive and often slow to recover from injury, making neurotoxicity and safety pharmacology testing critical.
iPSC-derived neurons offer a highly relevant human-based platform for detecting and studying these toxic effects before they reach patients, supporting early detection of neurotoxic risks such as disrupted neurotransmission, dendritic damage or cell death, which may not be evident in traditional animal studies.
Neurotoxicology Toxicology and Safety Pharmacology Assays
Ncardia’s customizable iPSC-based neurotoxicology and safety assay capabilities cover a spectrum of functional, structural and biochemical endpoints to detect subtle neural damage — improving the predictive power of safety evaluations:
- Assays can be multiplexed for functional and structural cardiotoxicity assessments
- Noninvasive assays allow both acute and chronic studies
| Proliferation, adhesion & cell loss | IncuCyte, ImageXpress Confocal, CardioExcyte 96, ECIS Z-Theta |
|---|---|
| Gene expression | BioRad Opus 384 qPCR, RNAseq, whole genome sequencing |
| Protein expression | Western Blot, Spark Multimode Microplate Reader, NovoCyte Flow Cytometer, ImageXpress Confocal, MSD MESO Sector 600MM |
| Metabolism | Spark Multimode Microplate Reader, NovoCyte Flow Cytometer, ImageXpress Confocal |
| E-phys (MEA) | Maestro MEA, LUMOS |
| Ca2+ transients | FDSS µCell |
| Metabolism assays | Spark Multimode Microplate Reader, NovoCyte Flow Cytometer, ImageXpress Confocal |
The Latest From Ncardia

Webinar: Advancing the Future of Cardiac Safety
Get an exclusive look at next-generation approaches for greater confidence in preclinical testing, including Ncardia’s first-of-its-kind Ncyte® NHP-C vCardiomyocytes.
Use iPSC-Derived Neural Assays to Optimize Safety Pipelines
When used in drug discovery, iPSC-based neural assays enable more predictive, patient-specific evaluations, reduce reliance on less-representative models and support the development of safer therapeutics and chemicals.
- Ncardia has developed a translational calcium assay for predicting in vivo toxicity based on iPSC derived human neuronal co-cultures.
- Acute tolerability scores in mice correlate highly with calcium oscillation scores in human iPSC derived neurons (r2 > 0.8).
- Assay is robust and scalable (384 well format) to enable assessment of your ASOs at any stage of the drug discovery process.
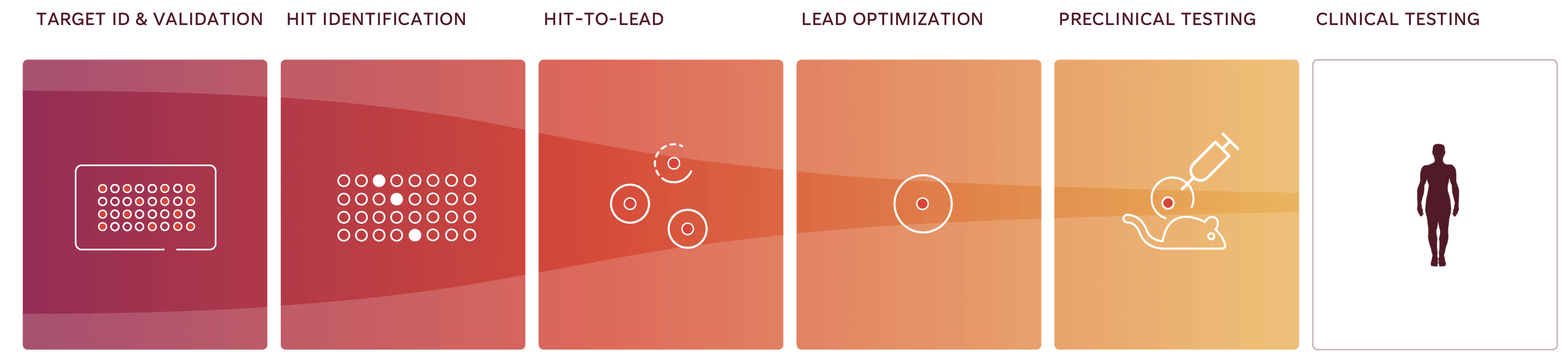
Partner With the Proven iPSC Experts
Are you ready to confidently advance your therapeutic candidates?
Start a Conversation