Ncyte® Microglia
Human iPSC-derived Microglia
Ncyte hiPSC-derived Microglia are high-purity microglial cells that demonstrate phagocytosis and cytokine release, closely mimicking the behavior of human microglia. They offer researchers a reliable, accessible, and physiologically relevant platform to study microglial interactions, responses to central nervous system injuries, and disease mechanisms.
Especially suited for co-culture and neuroinflammation studies, they support the advancement of therapeutic research and development for CNS disorders and a range of neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and ALS.
- Rapidly evaluate therapeutic compounds
- Effectively mediate inflammatory responses
- Valuable for investigating cytokine release in neuroinflammatory conditions
Do you want to buy Ncyte® Microglia?
To proceed with your order, please review and accept our Terms and Conditions above.
Do you need a larger volume or a custom cell model?
Product Specifications
Identity markers
≥80% TREM2+/CD11b+, ≥90% CD45+/CD46+, ≥70% IBA1 at thawing, according to user guide
Size
≥ 1.5 M viable cells at thawing, according to user guide
Quality Control
Cell count, Viability, Identity (FACS, ICC), Cytokine release, Mycoplasma
Format
Cryopreserved cells
Donor
Female
Reprogramming method
Non-viral
Shipping conditions
Dry shipper, -180°C to -135°C
Storage conditions
Vapor phase of liquid nitrogen
Technical data
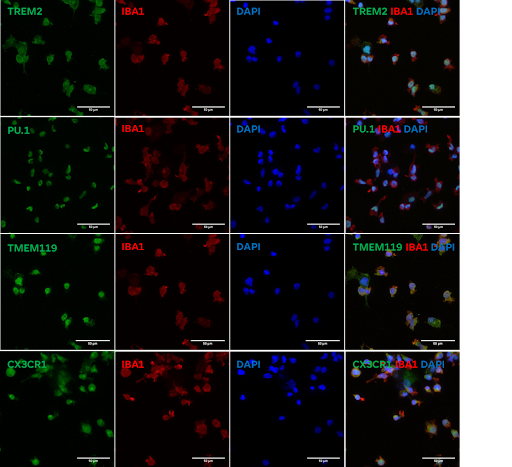
- Immunofluorescence staining demonstrates robust expression of microglial markers including TREM2, IBA1, PU.1, TMEM119, and CX3CR1 in Ncyte® Microglia.
- Confocal microscopy reveals co-localization of microglial markers, indicating the presence of a mature microglial phenotype.
- Ncyte® Microglia exhibit characteristic ramified morphology, further supporting their microglial identity.
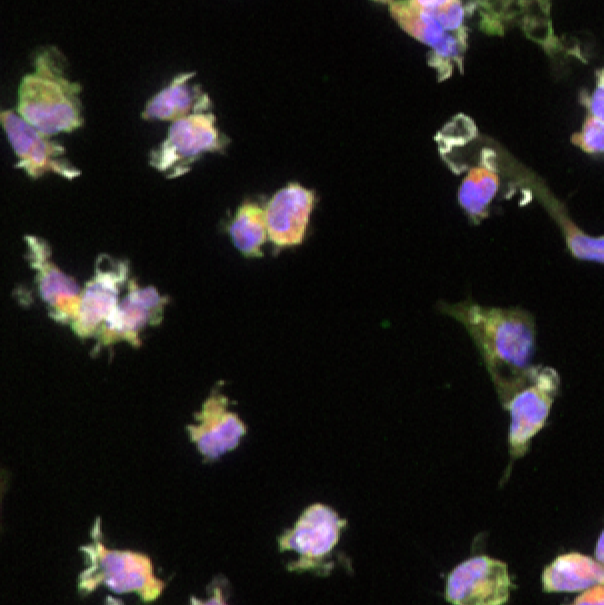
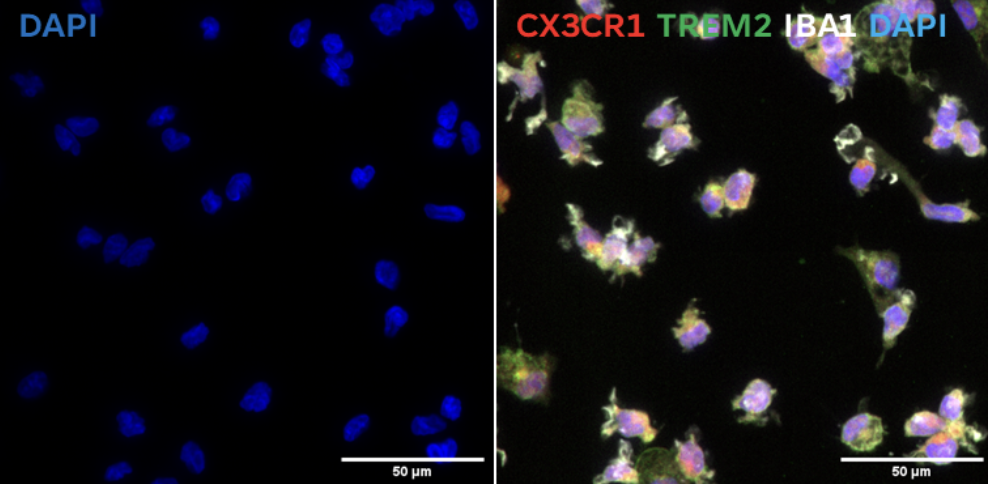
 Triple positive population for CX3CR1, TREM2, and IBA1 indicates mature microglial phenotype.
Triple positive population for CX3CR1, TREM2, and IBA1 indicates mature microglial phenotype.
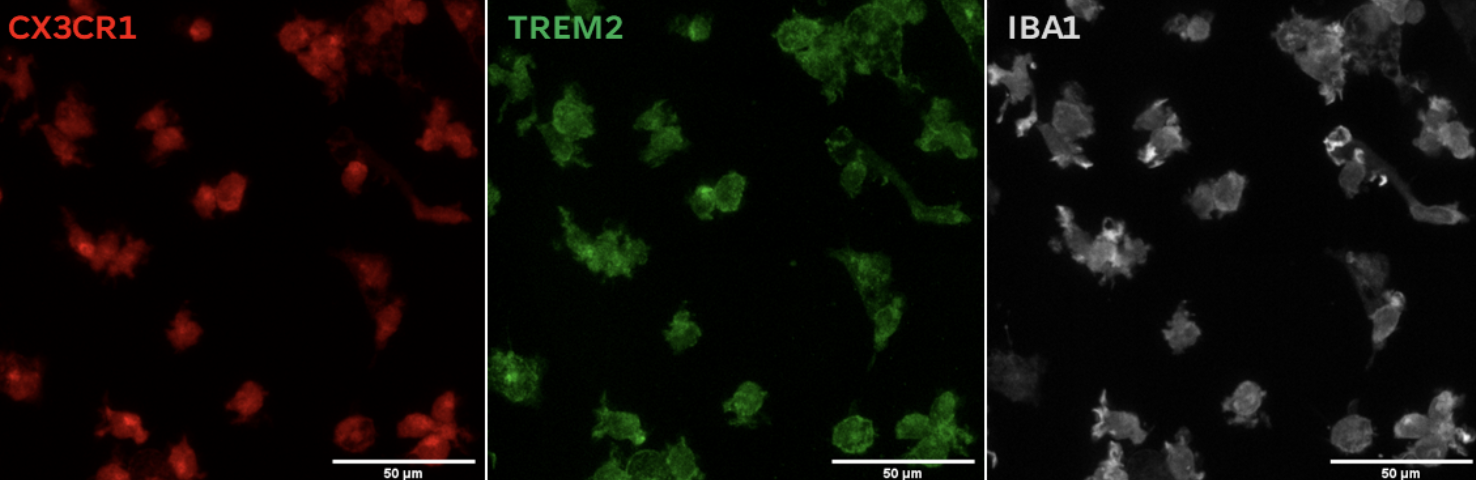
 Expression of CX3CR1 and TREM2 suggests suitability for co-culture systems with neurons to model Alzheimer's Disease and other neurodegenerative diseases.
Expression of CX3CR1 and TREM2 suggests suitability for co-culture systems with neurons to model Alzheimer's Disease and other neurodegenerative diseases.
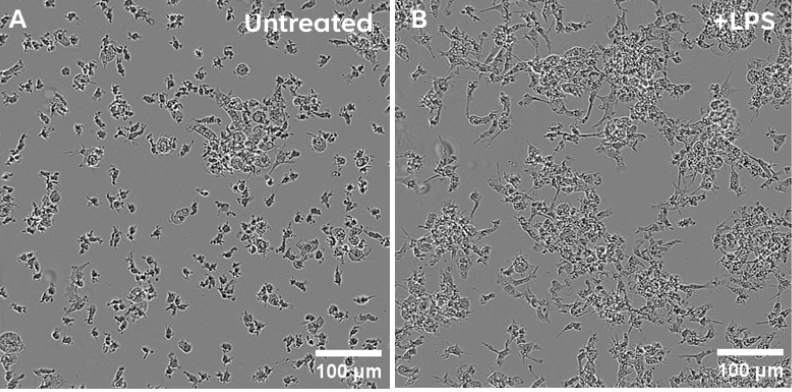 Brightfield images of Ncyte® Microglia on day 7 post-thaw. A) Untreated microglia and B) Microglia treated with LPS (100 ng/ml) for 18hrs.
Brightfield images of Ncyte® Microglia on day 7 post-thaw. A) Untreated microglia and B) Microglia treated with LPS (100 ng/ml) for 18hrs.
- Morphological evidence of activation is demonstrated by changes in Ncyte® Microglia morphology following LPS stimulation.
- Flow cytometry and ICC confirm robust expression of key microglial markers (CX3CR1, TREM2, IBA1, CD45, CD11b) in both resting and activated states.
- Ncyte® Microglia show significant cytokine release upon LPS stimulation, highlighting their functional role in mediating inflammatory responses.
- Ncyte® Microglia offer an ideal in vitro model for studying neuroinflammation and the mechanisms of neurodegenerative diseases, including
Alzheimer’s and Parkinson’s.
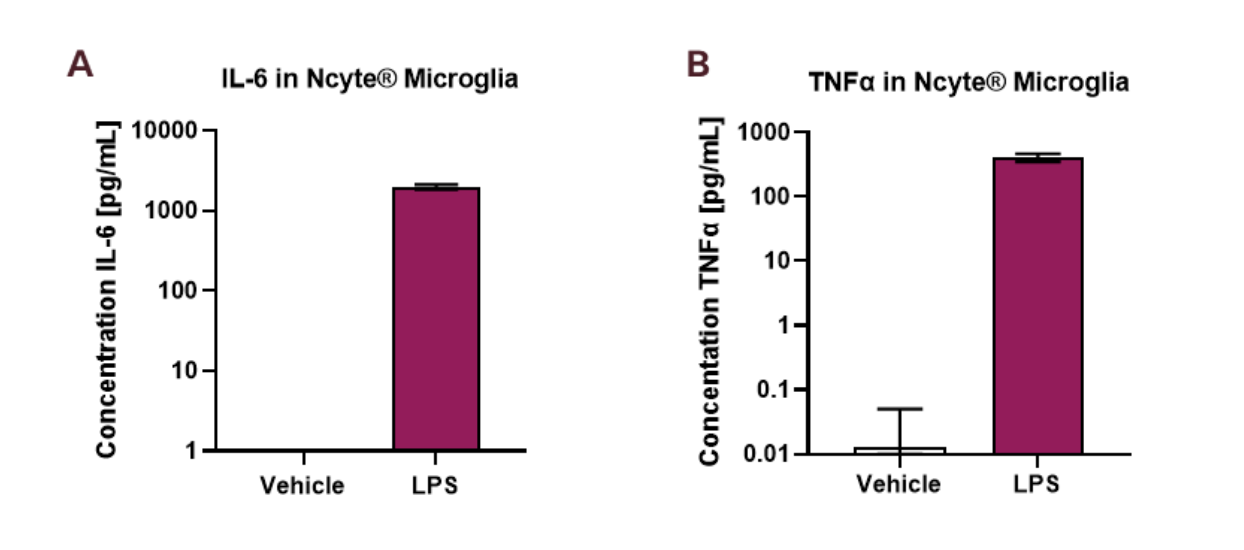
- Ncyte® Microglia exhibit a strong pro-inflammatory reaction upon LPS stimulation.
- LPS treatment significantly increases the production of IL-6 and TNF-α, key inflammatory mediators. These results underscore the ability of Ncyte® Microglia to effectively mediate inflammatory responses.
- Ncyte® Microglia provide a valuable in vitro model for investigating cytokine release in neuroinflammatory conditions.
Certificates of analysis are available upon request via support@ncardia.com
Our work centers on a simple yet powerful premise:
When we combine deep iPSC knowledge, broad assay capabilities and a demonstrated ability to integrate the biology of human diseases into preclinical research, we can help drug developers make critical decisions earlier and with more confidence.
Human iPSC-derived atrial cardiomyocytes
Neural
Human iPSC-derived astrocytes
Vascular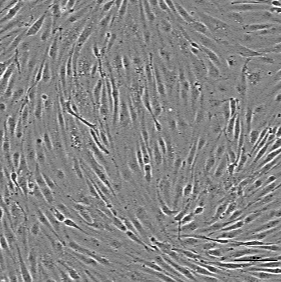
Human iPSC-derived vascular endothelial cells
Cardiac.png)
Human iPSC-derived 3D cardiac microtissue model
Neural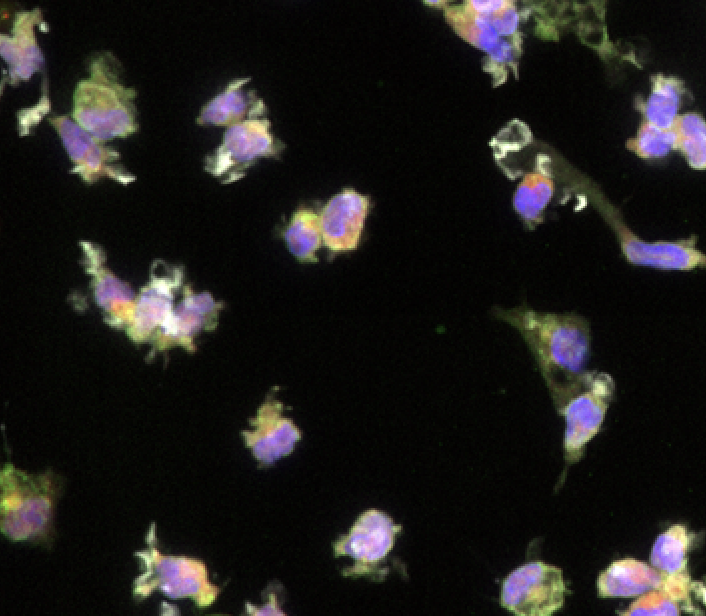
Human iPSC-derived microglia
Cardiac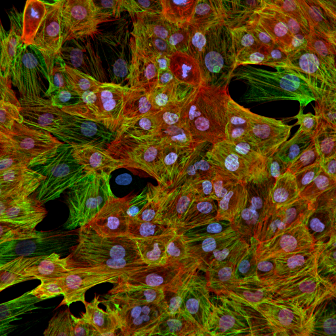
Non-Human Primate Cynomolgus iPSC-Derived Ventricular-Like Cardiomyocytes
Vascular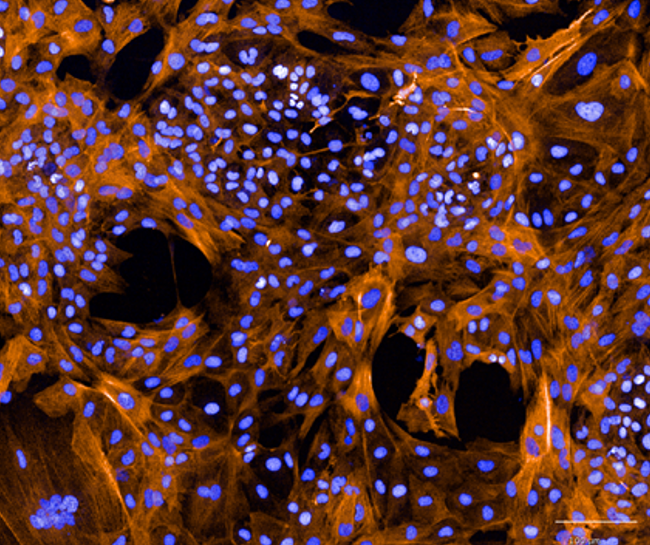
Human iPSC-derived vascular smooth muscle cells
Cardiac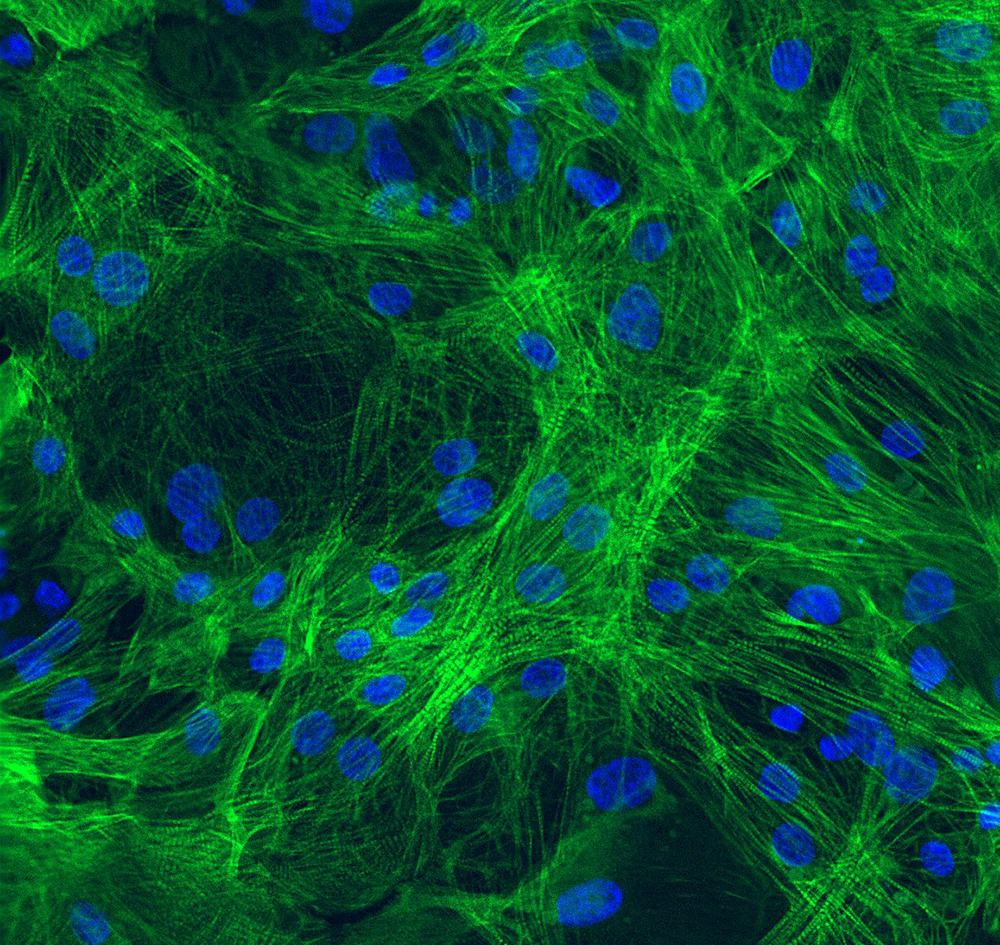
Human iPSC-derived ventricular cardiomyocytes
