Ncyte® vCardiomyocytes
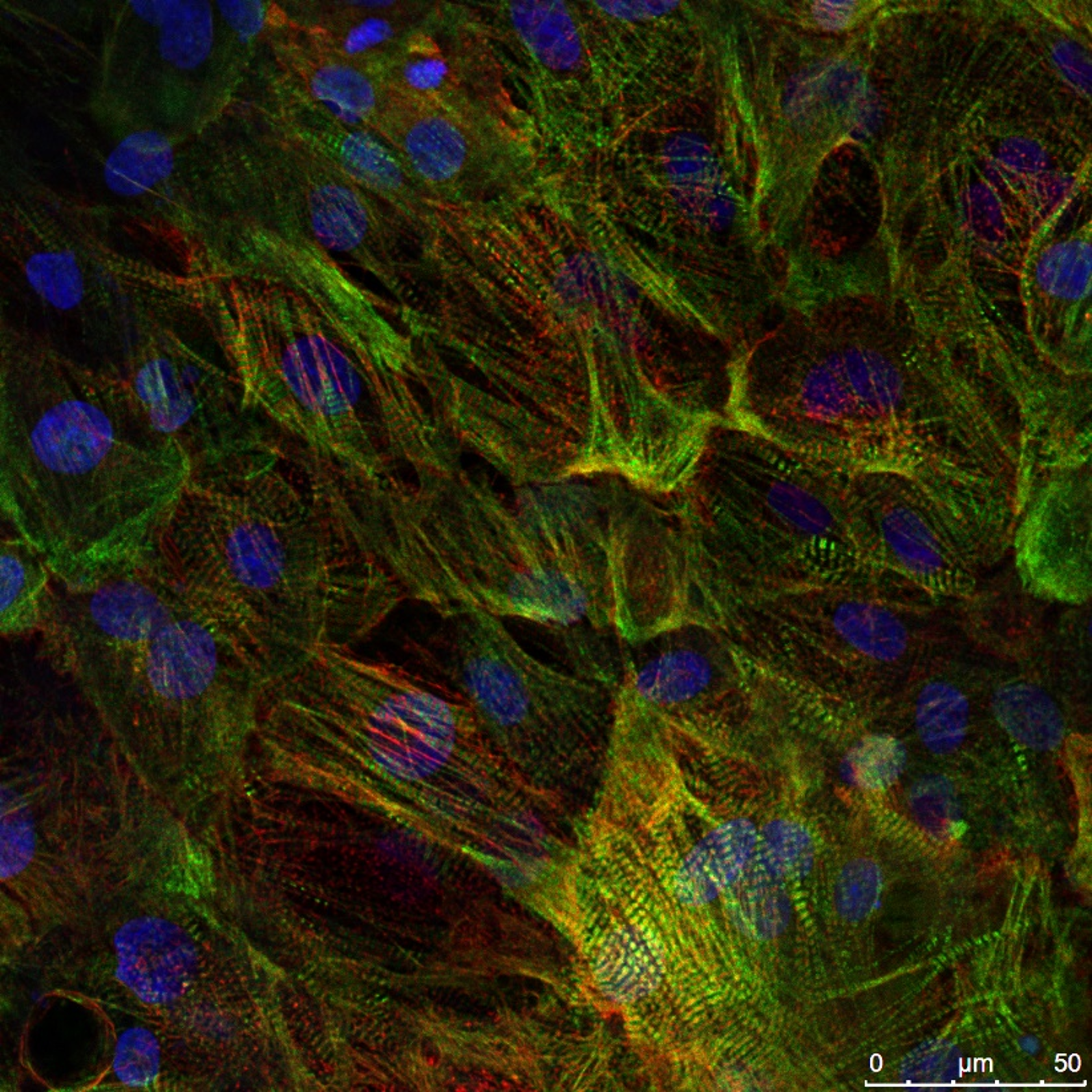
Ncyte® vCardiomyocytes are physiologically relevant, functional ventricular cardiomyocytes (vCMs) built from human iPSC-derived cells and validated across a robust set of key safety pharmacology and cardiotoxicity assays. Perfectly adaptable to all assays’ needs, our vCMs offer a scalable, reproducible, stable and consistent solution for confidently integrating human-relevant cardiac models into your research.
Whether you need our in-house screening services or a reliable cell supply, we provide flexible delivery volumes of up to 10 billion cells per batch to meet your screening campaign’s needs.