Ncyte® aCardiomyocytes
Human iPSC-derived atrial-like cardiomyocytes
Atrial cardiomyocytes play a crucial role in cardiac function, not just by contracting to pump blood, but also through their hormone production, electrical properties, and Ca2+ signaling.
Ncyte® aCardiomyocytes are a powerful tool to improve in vitro testing of new treatments for atrial arrhythmias. The high purity and consistency of this model can help you increase reproducibility of your results. The functionality of the cells enables it’s application in high-throughput assays for large-scale screening with minimal variability.
- Avoid lot-to-lot variability
- Functional and physiologically relevant
- Suitable for electrophysiological studies
Do you want to buy Ncyte® aCardiomyocytes?
To proceed with your order, please review and accept our Terms and Conditions above.
Do you want to buy Ncyte® Cardiomyocyte Culture Medium?
To proceed with your order, please review and accept our Terms and Conditions above.
Do you need a larger volume or a custom cell model?
Product Specifications
Identity markers
≥ 70% cTnT+ cells and ≥ 70% COUP-TFII+ cells from cTnT+ population at day 3 of culture according to user guide
Size
≥ 2M viable cells at day 3 of culture according to user guide
Quality Control
Cell count, viability, identity, human pathogen screening, mycoplasma
Format
Cryopreserved cells
Donor
Female
Reprogramming method
Ectopic expression of reprogramming factors using episomal plasmids
Shipping conditions
Dry shipper, -180°C to -135°C
Storage conditions
Vapour phase of liquid nitrogen
Optional Supply of
Ncyte® Cardiomyocytes Medium
Technical data
Ncyte® aCardiomyocytes are characterized by flow cytometry to ensure a high purity, assessing double expression of cardiac Troponin T (cTnT) and the specific COUP transcription factor II (COUP-TFII), as shown in the figure.
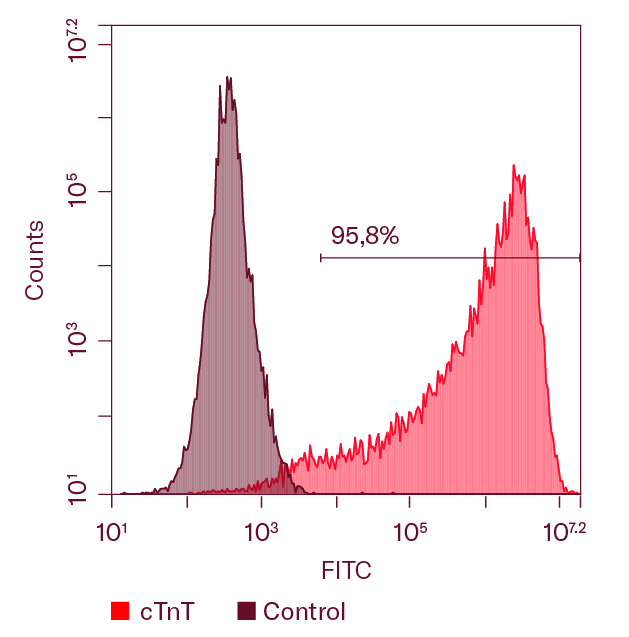 Flow cytometry analysis of one representative batch of Ncyte® aCardiomyocytes 3 days after thawing
Flow cytometry analysis of one representative batch of Ncyte® aCardiomyocytes 3 days after thawing
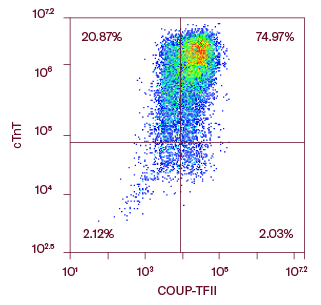 Flow cytometry analysis of one representative batch of Ncyte® aCardiomyocytes 3 days after thawing
Flow cytometry analysis of one representative batch of Ncyte® aCardiomyocytes 3 days after thawing
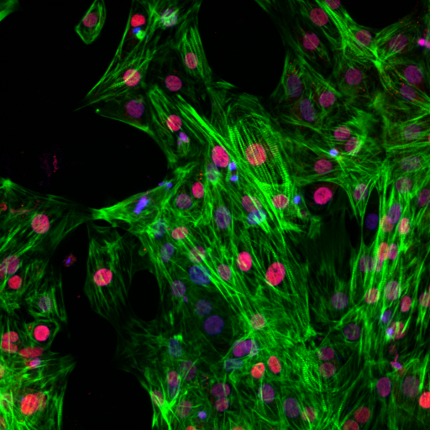
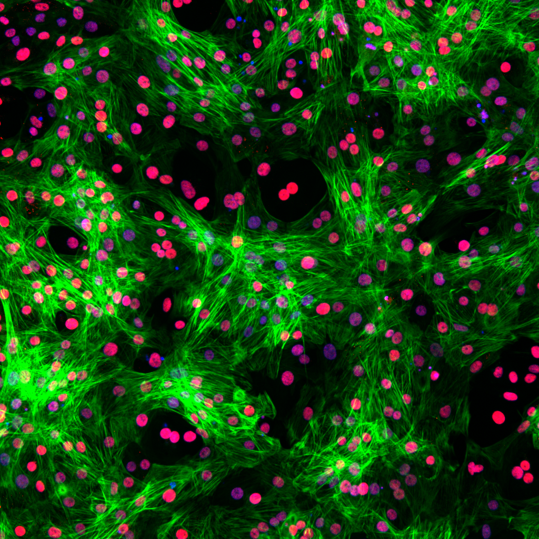 Immunofluorescence staining of Ncyte® aCardiomyocytes 3 days after thawing, showing double expression of cTnT (green) and the specific atrial marker COUP-TFII (red). DAPI (blue).
Immunofluorescence staining of Ncyte® aCardiomyocytes 3 days after thawing, showing double expression of cTnT (green) and the specific atrial marker COUP-TFII (red). DAPI (blue).
Ncyte® aCardiomyocytes show atrial-like action potentials and high electrical activity as measured by MEA analysis. They show a stable beat rate with very low inter- and intra-well variation. This model is, therefore, physiologically relevant for the phenotypic study of atrial arrhythmias.
Our expert scientists can develop and execute tailor assays to support your drug discovery research in the cardiac field.
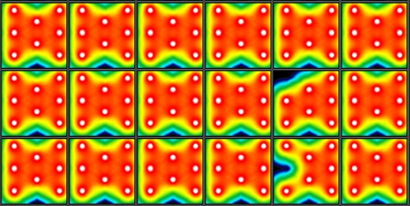 Ncyte® aCardiomyocytes in the multi-electrode array (MEA) recording system, showing near to complete electrode coverage in all wells with electrical activity >300 μV.
Ncyte® aCardiomyocytes in the multi-electrode array (MEA) recording system, showing near to complete electrode coverage in all wells with electrical activity >300 μV.
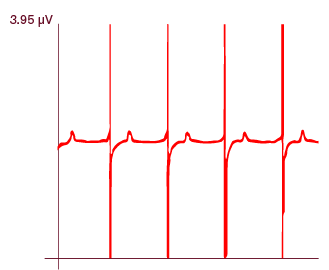 MEA analysis demonstrates that Ncyte® aCardiomyocytes present robust re- and depolarization peak amplitudes, critical for fast and accurate data analysis.
MEA analysis demonstrates that Ncyte® aCardiomyocytes present robust re- and depolarization peak amplitudes, critical for fast and accurate data analysis.
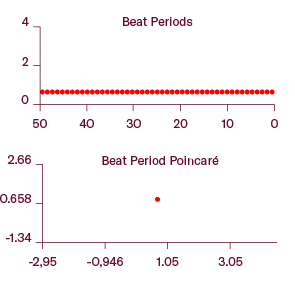 Analysis of over 50 beats demonstrate high stability and consistent beating patterns.
Analysis of over 50 beats demonstrate high stability and consistent beating patterns.
Ncyte® aCardiomyocytes are suitable for electrophysiological research and drug discovery. In this example, they show relevant pharmacological responses to known cardioactive compounds with acute and chronic treatments (culturing according to project needs).
Our scientists are also able to create and refine custom assays to evaluate the efficacy and safety of new therapeutic candidates using this model.
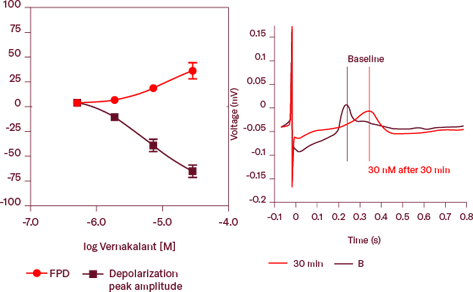 Concentration-dependent increase in field potential duration and decrease in depolarization peak amplitude after treatment with Vernakalant, a multi-channel blocker
Concentration-dependent increase in field potential duration and decrease in depolarization peak amplitude after treatment with Vernakalant, a multi-channel blocker
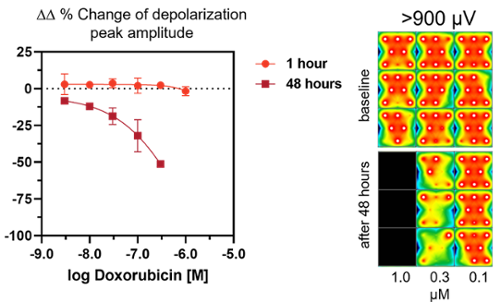 Chronic drug-induced cardiotoxicity: concentration- and time-dependent decrease of depolarization peak amplitudes and number of active electrodes after treatment with Doxorubicin
Chronic drug-induced cardiotoxicity: concentration- and time-dependent decrease of depolarization peak amplitudes and number of active electrodes after treatment with Doxorubicin
Certificates of analysis are available upon request via support@ncardia.com
Our work centers on a simple yet powerful premise:
When we combine deep iPSC knowledge, broad assay capabilities and a demonstrated ability to integrate the biology of human diseases into preclinical research, we can help drug developers make critical decisions earlier and with more confidence.

Human iPSC-derived atrial cardiomyocytes
Neural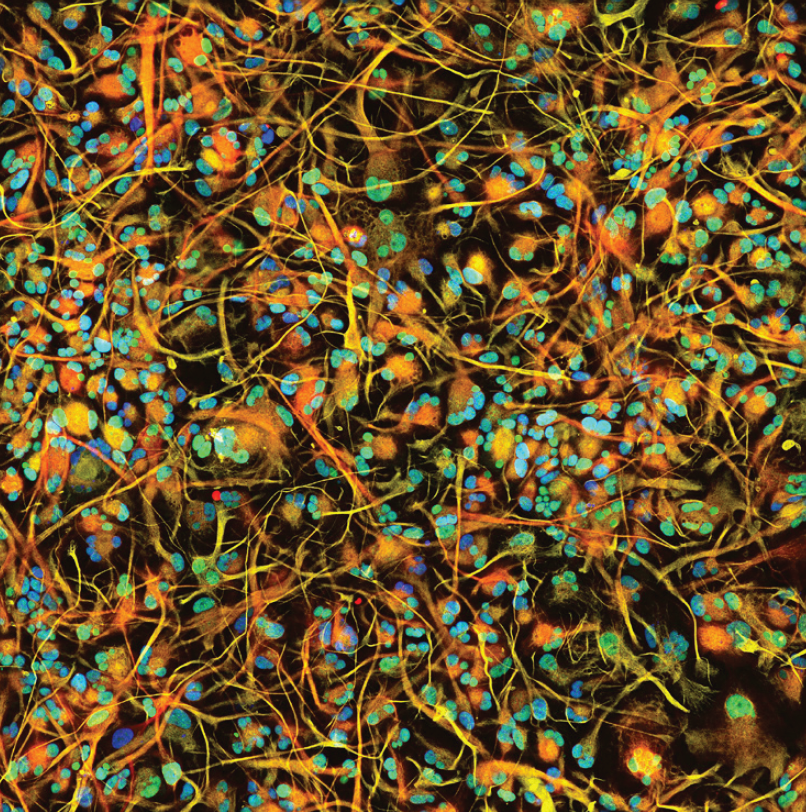
Human iPSC-derived astrocytes
Vascular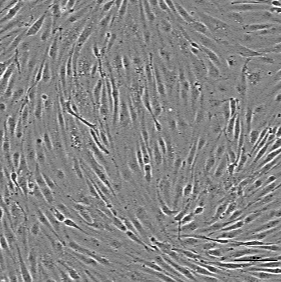
Human iPSC-derived vascular endothelial cells
Cardiac.png)
Human iPSC-derived 3D cardiac microtissue model
Neural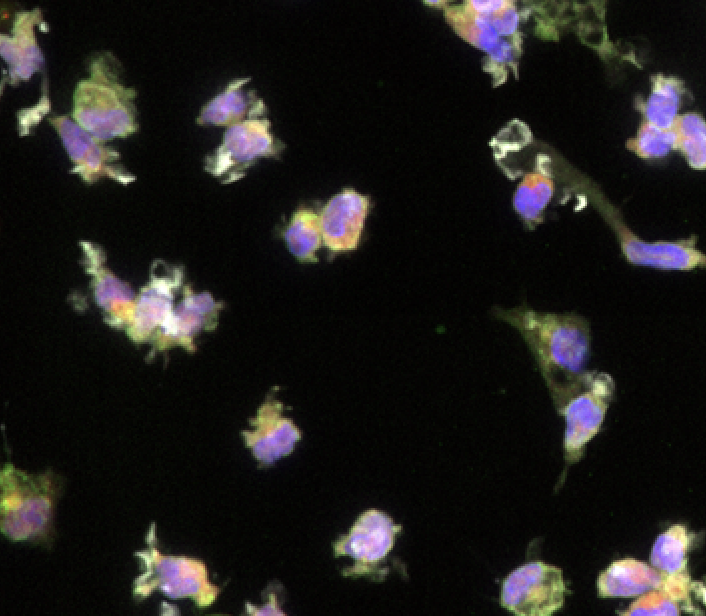
Human iPSC-derived microglia
Cardiac
Non-Human Primate Cynomolgus iPSC-Derived Ventricular-Like Cardiomyocytes
Vascular
Human iPSC-derived vascular smooth muscle cells
Cardiac
Human iPSC-derived ventricular cardiomyocytes


